Proteins
Proteins are one of the four main groups of macromolecules or polymers found in all living organisms. They play a wide range of roles essential to cellular functions. Proteins are formed through a series of condensation reactions between the carboxyl group of one amino acid and the amino group of another, resulting in peptide bonds. Most enzymes are proteins, highlighting their biological significance.
Sources of Proteins
Proteins are present in every living system, serving as structural components and as vital substances like hormones, enzymes, and pigments.
- First-class proteins: These contain all the essential amino acids and are mainly of animal origin. Examples include lean meat, fish, eggs, milk, cheese, and chicken.
- Second-class proteins: These are derived from plant sources and can be found in vegetables, legumes (e.g. beans, soya beans), groundnuts, grapes, apples, etc.
Examples of Proteins
- Keratin: Found in skin, hair, and nails
- Collagen: A muscle protein important for structural integrity
- Insulin: A peptide hormone that regulates blood sugar levels
- Elastin: A key component of elastic fibers
- Amylase: An enzyme in human saliva that breaks down starch into sugar
- Haemoglobin: A pigment in red blood cells that carries oxygen
Structure and Shape of Proteins
Proteins have distinct and organized structures. The primary structure refers to the specific sequence in which atoms are covalently bonded to form chains and cross-links between chains.
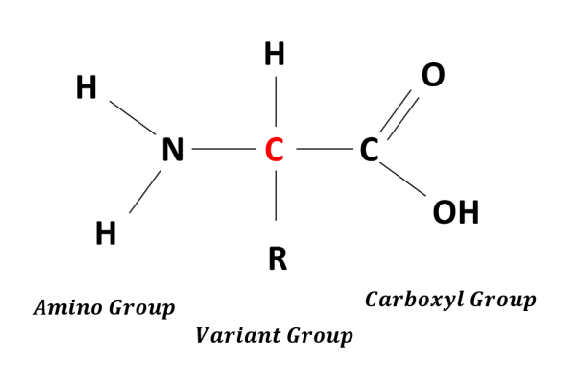
Amino acids are the building blocks of proteins. Each amino acid contains an amino group (-NH2) and a carboxyl group (-COOH). A protein may be composed of two or more different amino acids arranged in a specific sequence that is unique to that protein. Even if two proteins have the same types of amino acids, their sequences will differ, making each protein unique.
 Credit: Nadia Arrousse On researchgate
Credit: Nadia Arrousse On researchgate
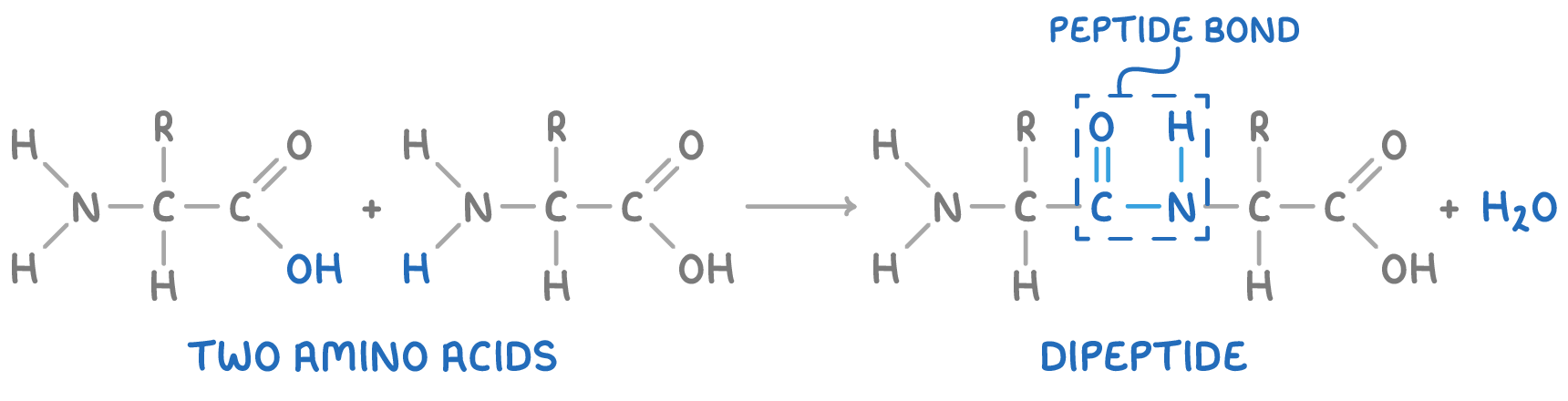
Formation of Peptides
Amino acids in a protein macromolecule are connected via peptide bonds. This bond forms when a molecule of water is eliminated between the carboxyl group of one amino acid and the amino group of another, resulting in an amide linkage known as a peptide bond.
 Credit: Cognito
Credit: Cognito
Types of Peptides
- Peptides: Formed by linking two amino acids
- Tripeptides: Formed by linking three amino acids
- Polypeptides: Long chains formed by linking many amino acids
This process continues as a chain reaction to form proteins with various lengths and complexities.
Properties of Proteins
- Solubility in Water: Most proteins are insoluble in water. However, some proteins such as insulin (a hormone) and pepsin (an enzyme) are water-soluble. Others may form colloidal solutions.
- Effect of Heat: Proteins like egg white (albumin), when dissolved in water, form colloidal solutions. Heating this solution above 50°C leads to coagulation or precipitation of the protein. This change is called denaturation.
- Hydrolysis: Hydrolysis breaks peptide bonds in proteins. When proteins are boiled with acids, alkalis, or enzymes, they are broken down into individual amino acids. These amino acids can be separated and identified using paper chromatography.
Tests for Proteins
- Biuret Test: Add a few drops (about 2 cm³) of sodium hydroxide (NaOH) solution to the protein sample in a test tube, followed by a few drops of copper(II) sulfate (CuSO₄) solution. A violet or purple coloration confirms the presence of protein.
- Millon's Test: Add 2 cm³ of Millon’s reagent to the protein solution in a boiling tube. A white precipitate forms, which turns brick-red upon heating, indicating the presence of protein.
- Trioxonitrate(V) Acid Test: Treat a protein or amino acid solution with concentrated nitric acid (HNO₃). A white precipitate forms and, upon heating, turns intense yellow, confirming the presence of protein.
Uses of Proteins
- Haemoglobin, a protein in the blood, transports oxygen and carbon dioxide.
- Hormones that regulate biological processes are made of proteins.
- Enzymes, which are proteins, catalyze biological reactions.
- Animal hides are tanned using proteins to produce leather used in shoes, bags, and belts.
- Casein, a milk protein, is used to make artificial wool and silk.
- Proteins are an essential class of food required for a balanced diet.
Enzymes
Enzymes are organic catalysts that facilitate metabolic reactions in living organisms. They are protein molecules secreted by living cells.
Hydrolases are enzymes involved in hydrolysis reactions, aiding in digestion. There are also oxidation-reduction enzymes such as oxidase, dehydrogenase, and oxygenase that help oxidize food in body cells.
Properties of Enzymes
- Enzymes are soluble in body tissues.
- They act specifically on their target substrates.
- They are temperature-sensitive, functioning best between 33°C and 40°C. Higher temperatures denature them.
- They are pH-sensitive, working best within specific pH ranges.
- Enzymes are very efficient and catalyze reactions rapidly.
How Enzymes Work
- The enzyme binds with the substrate to form an enzyme-substrate complex.
- Within this complex, the substrate reacts to form the enzyme-product complex.
- The complex then separates into the final product and the unchanged enzyme, which is ready for another cycle.