Carbohydrates
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen, with the hydrogen and oxygen atoms typically in a 2:1 ratio. Their general molecular formula is either CxH2yOy or Cx(H2O)y. Examples include water-soluble sugars like glucose and sucrose, as well as larger, insoluble carbohydrates such as starch, glycogen, and cellulose.
Sources of Carbohydrates
Common sources include rice, yam, maize, millet, guinea corn, potatoes, bread, and garri.
Classes of Carbohydrates
- Simple Sugars (Monosaccharides & Disaccharides): These are crystalline, water-soluble, and sweet. Examples include glucose, fructose, and sucrose.
- Complex Sugars (Polysaccharides): Also called polysaccharides, these are non-crystalline, insoluble, tasteless, and have high molecular masses. Examples include starch and cellulose.
 Credit: Marcin Horbowicz on
Researchgate
Credit: Marcin Horbowicz on
Researchgate
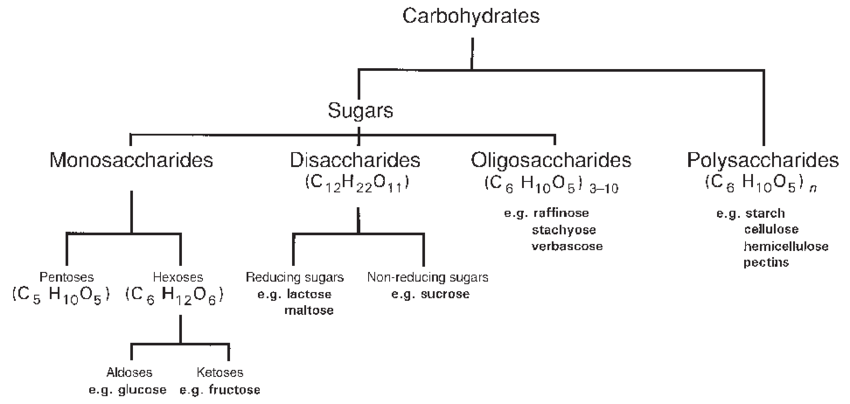
Classification Based on Hydrolysis
- Monosaccharides: Cannot be hydrolyzed into simpler sugars. Examples: Glucose, Fructose, Galactose.
- Disaccharides: Yield two monosaccharides upon hydrolysis. Examples: Sucrose, Lactose, Maltose.
- Polysaccharides: Hydrolyze into many monosaccharides. Examples: Cellulose, Starch, Glycogen.
A monosaccharide with an aldehyde group is called an aldose, while one with a ketone group is a ketose. Based on carbon atoms, they are called trioses, tetroses, pentoses, hexoses, etc. Glucose is an aldohexose, while fructose is a ketohexose.
 Credit: petrroudny43
Credit: petrroudny43
Reducing and Non-Reducing Sugars
Sugars that react with Fehling’s, Benedict’s, or Tollen’s reagent are known as reducing sugars. Most monosaccharides and many disaccharides (except sucrose) are reducing sugars.
Glucose
Glucose is a simple sugar found in grapes, honey, and plant sap. It is a key energy source for animal tissues and is formed during photosynthesis. It exists in open-chain and cyclic forms.
Laboratory Preparation of Glucose
By hydrolyzing sucrose with dilute acid and ethanol:
C12H22O11 + H2O → C6H12O6 + C6H12O6
Industrial Preparation of Glucose
By hydrolyzing starch with dilute acid under pressure:
(C6H10O5)n + nH2O → nC6H12O6
Properties of Glucose
- Strong reducing agent due to the –CHO group.
- Forms black carbon residue when heated with concentrated H2SO4.
- Ferments to ethanol and CO2 in the presence of zymase enzyme.
Test for Glucose
Add a few drops of Fehling’s solution to glucose and boil. A brick-red precipitate confirms its presence.
Uses of Glucose
- Used in making jams and sweets.
- Serves as a quick energy source for patients and athletes.
Fructose
Fructose (C6H12O6) is found in sweet fruits and honey, often with glucose. It differs in its oxidation behavior: when oxidized with HNO3, it forms acids with fewer than six carbon atoms.
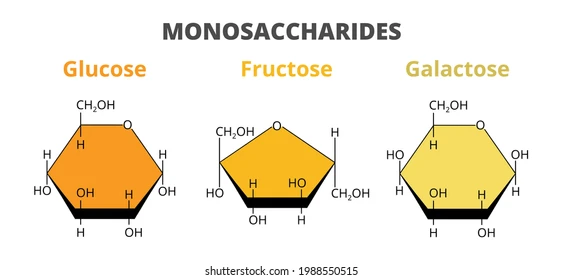
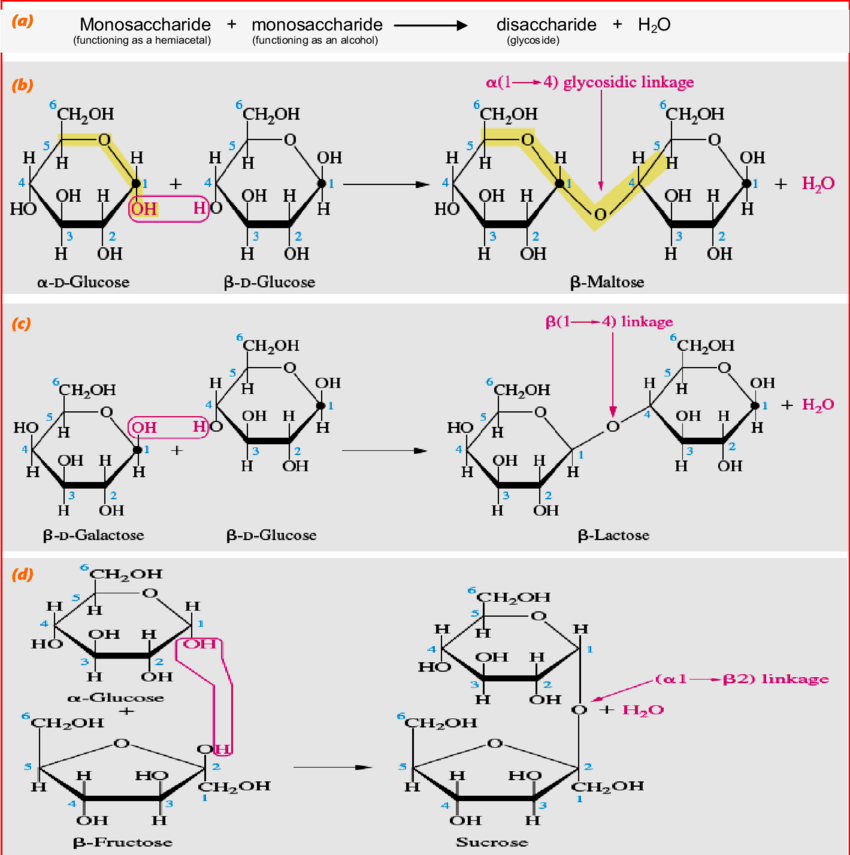
Disaccharides
Disaccharides form from two monosaccharides via condensation, releasing one water molecule. Their general formula is C12H22O11.
 Credit: Demeiape Bagalay on
reseachgate
Credit: Demeiape Bagalay on
reseachgate
Common Disaccharides
- Glucose + Fructose → Sucrose
- Glucose + Galactose → Lactose
- Glucose + Glucose → Maltose
Sucrose
Sucrose is the common table sugar from sugar cane or beet.
Preparation
Extracted with warm water (~80°C), purified using slaked lime and CO2, then concentrated by evaporation.
Physical Properties
- Colourless, crystalline, very sweet.
- Soluble in water, not in ethanol.
Chemical Properties
- Chars when heated with concentrated H2SO4.
- Forms caramel when heated to 210°C.
- Easily hydrolyzed by dilute acid or enzymes.
Test for Sucrose
Add freshly prepared Seliwanoff reagent to 5 cm3 of sucrose solution and warm. A red color appears after 10 minutes.
Uses of Sucrose
- Sweetening food
- Preserving food
- Fermenting to produce ethanol
- Caramel used in flavouring and confectionery
Maltose
Maltose (C12H22O11) is formed from starch by malt, which contains diastase enzyme.
Properties
- White, crystalline, and soluble
- Melts at 160–165°C
- Hydrolyzed by dilute acids or maltase
- Reducing sugar
Lactose
Lactose (C12H22O11) is found in milk but not plants.
Properties
- White, crystalline, and soluble
- Melts at 203°C
- Hydrolyzed to glucose and galactose by dilute acids or lactase
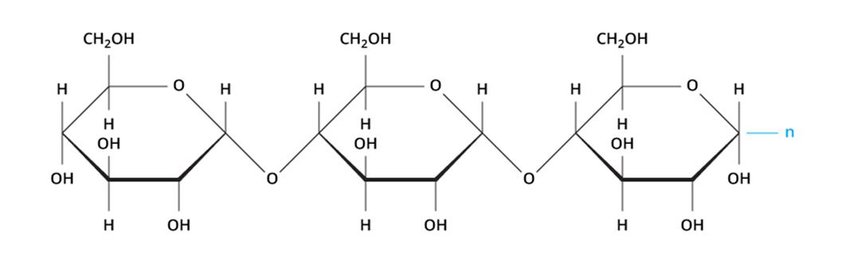
Polysaccharides
Polysaccharides are large polymers of monosaccharides joined by condensation. Their molecular weights range from thousands to millions. Examples include glycogen, cellulose, starch, and inulin.
Starch
Preparation of Starch
Peel and crush raw materials like cassava. Mix with water to extract starch. Let it settle, then decant water to leave starch residue.
Properties of Starch
- White, odorless, tasteless powder
- Insoluble in cold water but forms jelly in hot water
- Hydrolyzed by dilute acids to maltose and glucose
- Decomposed by diastase to maltose
- Not a reducing sugar
 Credit: Disha Trivedi on
Researchgate
Credit: Disha Trivedi on
Researchgate
Test for Starch
Add iodine to boiled starch. A blue-black color appears, which disappears on heating and returns on cooling.
Uses of Starch
- Used as food
- Used in ethanol and glucose production
- Used as a stiffening agent
Cellulose
Cellulose is the main structural component of plant cell walls and fibers. Found in cotton and wood, it's the most abundant organic substance.
Properties of Cellulose
- White solid, insoluble in water and most organic solvents
- Resistant to hydrolysis by dilute acids
- Hydrolyzed by cellulase enzyme
Uses of Cellulose
- Used in paper, textiles, ropes, and cellophane
- Also used in making gum, cotton, and explosives
Garri Production
To make garri, cassava is peeled, washed, and grated to a mash. Optional palm oil is added (for oil garri). The mash is pressed to remove water, then sieved and fried in a hot pan. The dry granules are stored or ground to fine flour.