Amines and Amides
Amines
Amines are organic compounds derived from ammonia (NH3) by replacing one or more hydrogen atoms with alkyl or aryl groups. They are functional groups characterized by a nitrogen atom with a lone pair of electrons. Amines have a general molecular formula of RNH2 and can form up to three bonds like ammonia, giving them a pyramidal shape due to sp3 hybridization.
They naturally occur in proteins, vitamins, and hormones, and are also synthetically prepared for use in dyes, drugs, and polymers.
Examples include methylamine (CH3NH2), ethylamine (C2H5NH2), and aniline (C6H5NH2).
Alkyl & Aromatic Amines
Alkylamines
These contain tetrahedral nitrogen centers. The C–N bond length is slightly shorter than a C–C bond. Alkylamines can be chiral due to the lone pair on nitrogen and the different groups attached.
Aromatic Amines
In aromatic amines like aniline, nitrogen adopts a planar structure due to delocalization of the lone pair with the aryl ring. The C–N bond is shorter due to partial double-bond character.
Naming of Amines
Amines are named by replacing the suffix “e” in the parent hydrocarbon name with “amine.”
- Primary amine: Methylamine (CH3NH2)
- Secondary/Tertiary: Named with “di-” or “tri-” prefixes (e.g., dimethylamine, trimethylamine)
- Aromatic amines: Named as derivatives of aniline, e.g., N,N-dimethylaniline
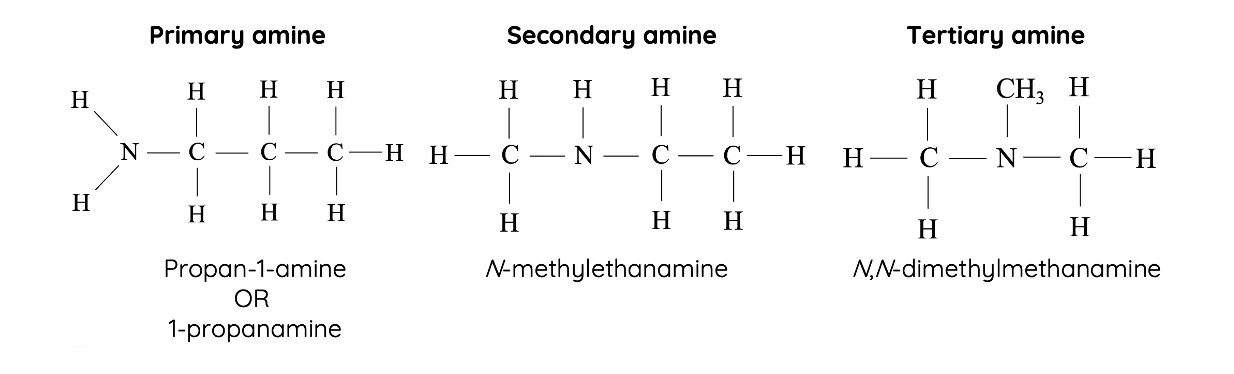
Classification of Amines
- Primary (1°): One hydrogen replaced. e.g., CH3NH2
- Secondary (2°): Two hydrogens replaced. e.g., (CH3)2NH
- Tertiary (3°): All three hydrogens replaced. e.g., N(CH3)3
 Credit: scienceready
Credit: scienceready
Cyclic amines such as aziridine and piperidine fall under secondary or tertiary amines depending on substitution.
Preparation of Amines
1. From Haloalkanes
Haloalkanes react with ammonia in ethanol under sealed conditions:
CH3CH2Br + NH3 → CH3CH2NH3+Br−
Then, the salt reacts with excess ammonia:
CH3CH2NH3+Br− + NH3 → CH3CH2NH2 + NH4Br
2. Alkylation of Alcohols
Alcohols react with ammonia to give amines:
ROH + NH3 → RNH2 + H2O
3. Gabriel Phthalimide Synthesis
Used to prepare primary amines by heating potassium phthalimide with alkyl halides and hydrolyzing the product. Not suitable for aromatic amines.
4. Benzoylation
Methylamine reacts with benzoyl chloride:
CH3NH2 + C6H5COCl → CH3NHCOC6H5 + HCl
5. Reduction of Nitriles
Nitriles are reduced with LiAlH4 to give primary amines.
6. Reductive Amination
Aldehydes or ketones react with ammonia or amines and are reduced to form amines.
7. Reduction of Amides
Amides are reduced using LiAlH4 to form amines:
- Primary amides → Primary amines
- N-substituted amides → Secondary or tertiary amines
Physical Properties of Amines
- Lower amines have fishy odour.
- Lower ones are gases; higher ones are liquids or solids.
- Aliphatic amines are more basic than aromatic ones.
- Boiling points increase with molar mass.
- They turn red litmus paper blue.
- Aryl amines like aniline oxidize and darken on exposure to air.
Chemical Properties of Amines
1. Dissociation in Water
They ionize to form hydroxide ions:
CH3NH2 + H2O → CH3NH3+ + OH−
2. Basicity
Amines react with acids to form salts. These can be regenerated with NaOH:
CH3NH3+Cl− + NaOH → CH3NH2 + NaCl + H2O
3. Alkylation
Amines react with alkyl halides via nucleophilic substitution to give higher amines.
4. Sulphonation
Aniline reacts with concentrated H2SO4 to form sulphanilic acid upon heating.
5. Acylation
Primary and secondary amines react with acyl chlorides or anhydrides in the presence of pyridine:
RNH2 + R'COCl → RNHCO–R' + HCl
Uses of Amines
- Production of polyamides (e.g., nylon)
- Manufacture of dyes and pigments
- Used as corrosion inhibitors and water treatment agents
- Key ingredients in analgesics (e.g., morphine)
- Used in insecticides and pesticides
- Important in producing amino acids and synthetic fibers
- Used as developers in photography
Amides
An amide is a functional group containing a carbonyl group (C=O) attached to a nitrogen atom. Amides are derived from carboxylic acids and have the general formula RCONH2 or RCONR2', where R and R' can be hydrogen or organic groups such as alkyl or phenyl.
In amides, the nitrogen atom forms single bonds with either hydrogen or carbon atoms. Naming amides involves replacing the "-ic" suffix of the acid with "amide".
- Acetic acid → Acetamide
- Ethanoic acid → Ethanamide
Nomenclature of Amides
- The root name is based on the longest carbon chain including the carbonyl group.
- Amides are derived by replacing the -OH of carboxylic acids with -NH2.
- The suffix "-e" of alkanes is replaced by "amide".
- Substituents on nitrogen are indicated with "N-" prefix.
Examples:
- Methanamide (HCONH2 or CH3NO)
- Ethanamide / Acetamide (CH3CONH2 or C2H5NO)
- Propanamide (CH3CH2CONH2 or C3H7NO)
- Butanamide (CH3CH2CH2CONH2 or C4H9NO)
Carbamide / Urea
Urea (Carbamide) is an organic compound with the formula CO(NH2)2. It contains two –NH2 groups joined by a carbonyl (C=O) group. Urea is the main nitrogenous waste in mammalian urine and plays a major role in nitrogen metabolism.
It is used in fertilizers and as a raw material in the chemical industry.
Classification of Amides
Primary Amides
Contain the group RCONH2. The nitrogen is bonded to two hydrogen atoms.
Examples: Methanamide (HCONH2), Ethanamide (CH3CONH2), Benzamide (C6H5CONH2)
Secondary Amides
Contain the group RCONHR'. The nitrogen is bonded to one hydrogen and one carbon group. Named using the prefix "N-" for the nitrogen substituent.
Examples: N-methylpropanamide (CH3CH2CONHCH3), N-phenylpropanamide (CH3CH2CONHC6H5)
Tertiary Amides
Contain the group RCONR'R". Nitrogen is bonded to two carbon atoms with no hydrogen. Named using "N,N-" prefix.
Examples: N,N-dimethylformamide (HCON(CH3)2), N-ethyl-N-methylethanamide (CH3CON(CH3)C2H5)
Preparation of Amides
1. From Acyl Chlorides
Acid chlorides (RCOCl) react with ammonia to form amides:
CH3COCl + 2NH3 → CH3CONH2 + NH4Cl
2. From Ammonium Salts
Carboxylic acids react with ammonium carbonate to form ammonium salts, which dehydrate to amides upon heating:
CH3COOH + (NH4)2CO3 → CH3COONH4 + H2O + CO2
CH3COONH4 → CH3CONH2 + H2O
Physical Properties of Amides
- Formamide is a liquid; others are crystalline solids.
- Form hydrogen bonds.
- Lower aliphatic amides are water-soluble.
- High boiling and melting points.
- Polar in nature.
Chemical Properties of Amides I
1. Hydrolysis
In acidic or basic conditions, amides hydrolyze to carboxylic acids and ammonia:
RCONH2 + H2O → RCOOH + NH3
CH3CH2CONH2 + H2O → CH3CH2COOH + NH3
2. Hofmann Degradation
Amides react with Br2 and NaOH to give primary amines with one carbon less:
RCONH2 + Br2 + 4NaOH → RNH2 + Na2CO3 + 2NaBr + 2H2O
3. Amphoteric Character
Amides act as both acids and bases:
As Acid: 2CH3CONH2 + HgO → (CH3CONH2)2Hg + H2O
As Base: CH3CONH2 + HCl → CH3COCl + NH3
Chemical Properties of Amides II
4. Dehydration
Amides are dehydrated to nitriles using phosphorus(V) oxide:
RCONH2 → RCN + H2O
5. Reaction with Nitrous Acid
Amides react with nitrous acid to form carboxylic acids and nitrogen gas:
NaNO2 + HCl → NaCl + HNO2
CH3CONH2 + HNO2 → CH3COOH + N2 + H2O
6. Reduction
Amides are reduced to primary amines using lithium aluminium hydride:
RCONH2 + 4[H] → RCH2NH2 + H2O
Uses of Amides
- Used in rubber, paper, plastics, crayons, pencils, and ink industries.
- Polyacrylamide is used in water treatment.
- Acrylamide is used in coatings for appliances and vehicles.
- Used to produce primary amines.
- Used in cosmetics: soaps, lotions, hair products.
- Used in explosives, adhesives, emulsions, and inks.
Test for Amides
1. Test with Alkali
When heated with sodium hydroxide, amides release ammonia (ammoniacal smell).
2. Nitrous Acid Test
Amides heated with HCl and NaNO2 cause effervescence due to nitrogen gas release.
3. Burning Test
Amides burn with a non-sooty flame and leave a white residue.