Hydrocarbons
Alkanes
Alkanes are aliphatic hydrocarbons with the general molecular formula CnH2n+2. For example, when n = 5, the compound is pentane (C5H12). Alkanes do not contain any functional groups.
IUPAC Nomenclature for Aliphatic Compounds
In IUPAC naming, organic compounds are named using three parts: the root, suffix, and prefix.
- Root: Refers to the name of the parent hydrocarbon with the longest continuous carbon chain.
- Suffix: Refers to the principal functional group attached to the longest chain.
- Prefix: Refers to any other substituents on the longest chain that are not the main functional group.
Example: In 1-chloroethane-1-ol, "1-chloro" is the prefix, "ethane" is the root, and "1-ol" is the suffix.
Rules for IUPAC Naming
- Select the longest continuous carbon chain as the parent hydrocarbon.
- Number the chain from the end nearest to the functional group.
- Use numbers and prefixes to indicate the positions and names of substituents.
- Use di-, tri-, tetra-, etc., if a substituent appears more than once.
- List different alkyl groups in alphabetical order.
- If halogens are present with alkyl groups, name halogens first (also in alphabetical order).
Methane (CH4)
Laboratory Preparation
Methane can be prepared in the lab by heating an ethanoate salt with soda lime (a mixture of sodium hydroxide and quicklime). Soda lime is preferred over caustic soda because it is less corrosive and does not absorb moisture from the air.
Physical Properties of Methane
- Colourless and odourless gas
- Slightly soluble in water
- Less dense than air
- Neutral to litmus paper
Chemical Properties of Methane
-
Combustion: Methane burns in oxygen
to produce carbon dioxide, water, and heat.
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) General: CₓHᵧ + (x + y/4)O₂ → xCO₂ + (y/2)H₂O -
Substitution Reaction: Methane
reacts with chlorine in the presence of UV light to
produce chloromethane and hydrogen chloride.
CH₄(g) + Cl₂(g) → CH₃Cl(g) + HCl(g)
Uses of Methane
- Used as a fuel, either alone or in combination with other gases
- Used in the production of hydrogen gas
- Used in the manufacture of carbon black
- Trichloromethane (a derivative) is used as an anaesthetic in surgery
Isomerism
Isomerism is the phenomenon where compounds have the same molecular formula but different structural arrangements.
Types of Isomerism
- Structural Isomerism: Compounds differ in the arrangement of atoms or bonding pattern.
- Stereoisomerism: Compounds have the same bonding arrangement but differ in spatial orientation.
Types of Structural Isomerism
- Chain Isomerism: Due to variations in the carbon chain structure.
- Functional Isomerism: Caused by differences in the functional group present.
- Positional Isomerism: Functional groups are located at different positions on the carbon chain.
Types of Stereoisomerism
- Geometric Isomerism: Compounds with the same molecular formula but different spatial arrangements due to double bonds or ring structures.
- Optical Isomerism: Compounds that differ in the way they rotate plane-polarized light due to the presence of chiral centers.
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are compounds in which carbon atoms are connected by multiple bonds. These bonds can be:
- Double bonds — found in alkenes
- Triple bonds — found in alkynes
Nomenclature
Alkenes are named by replacing the “ane” in alkanes with “ene.” For example:
- Ethane → Ethene
- Propane → Propene
Laboratory Preparation of Ethene
Ethene is prepared by heating ethanol with excess concentrated tetraoxosulphate(VI) acid (H2SO4) at 170°C. The acid acts as a dehydrating agent, removing water from the ethanol—a process called dehydration.
Reaction Steps:
-
C2H5OH(aq) + H2SO4(aq) → C2H5HSO4(aq) + H2O(l) -
C2H5HSO4(aq) → C2H4(g) + H2SO4(aq)
Overall Reaction:
C2H5OH(aq) —(H2SO4, 170°C)→ C2H4(g) + H2O(l)
Physical Properties of Ethene
- Colourless gas with a faint sweetish smell
- Slightly soluble in water
- Less dense than air
- Neutral to litmus paper
Chemical Properties of Ethene
-
Combustion: Ethene burns in air or
oxygen to produce carbon dioxide and water.
C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l) -
Addition Reactions: Two molecules
combine to form one molecule.
- Hydrogenation (Reaction with Hydrogen): Ethene reacts with hydrogen in the presence of a catalyst (like nickel) to form ethane.
-
Reaction with Halogens
(Halogenation):
Ethene reacts with halogens (e.g., bromine) to form dihalogenated compounds.
C2H4(g) + Br2(aq) → C2H4Br2(l)
This reaction decolourises bromine water, making it a test for unsaturation.
-
Reaction with Hydrogen Halides
(Hydrohalogenation):
Ethene reacts with hydrogen halides to form haloalkanes.
C2H4(g) + HCl(g) → C2H5Cl(l)
-
Reaction with Acidified or Alkaline
KMnO4 (Hydroxylation):
Ethene decolourises acidified KMnO4, forming ethane-1,2-diol.
C2H4(g) + [O] → CH2OH–CH2OH
In alkaline KMnO4, the purple solution turns green, also forming ethane-1,2-diol.
-
Reaction with Hydrogen Peroxide and Osmium
Tetroxide:
Ethene reacts with H2O2 in the presence of OsO4 to form ethane-1,2-diol.
C2H4(g) + H2O2(aq) —(OsO4)→ CH2OH–CH2OH
-
Reaction with Concentrated
H2SO4:
Ethene reacts with conc. tetraoxosulphate(VI) acid to form ethyl hydrogen sulphate.
C2H4(g) + H2SO4(l) → C2H5HSO4(l)
On hydrolysis:
C2H5HSO4(l) + H2O(l) → C2H5OH(aq) + H2SO4(aq)
-
Reaction with Bromine Water:
Ethene reacts with bromine water to form bromoethanol.
C2H4(g) + Br2(aq) + H2O(l) → CH2BrCH2OH(aq)
-
Polymerization:
Ethene molecules join to form polyethene (a plastic material).
nC2H4 → (–CH2–CH2–)n
-
Oxidation to Epoxyethane:
In the presence of silver catalyst at ~250°C, ethene reacts with oxygen to form epoxyethane.
C2H4(g) + ½O2(g) —(Ag, 250°C)→ C2H4O(g)
Uses of Ethene
- Used in the manufacture of plastics (polyethene)
- Used to produce synthetic rubber
- Accelerates ripening of fruits
- Used in the synthesis of various organic compounds like ethanol and haloalkanes
Nomenclature
Alkynes are a homologous series of unsaturated hydrocarbons with the general molecular formula CnH2n–2. They have a higher degree of unsaturation than alkenes, making them more chemically reactive than corresponding alkenes or alkanes.
They are named by replacing the suffix “-ane” of alkanes with “-yne”. For example, the first member of the alkyne series is ethyne, which has the molecular formula C2H2 and the structural formula HC≡CH.
Laboratory Preparation
Ethyne is prepared in the laboratory by reacting calcium carbide with cold water. This reaction is carried out on a bed of sand to prevent the reaction vessel from cracking due to the intense heat produced.
CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH)2(aq)
Physical Properties of Ethyne
- Ethyne is a colourless gas with a sweetish smell when pure.
- It is only sparingly soluble in water.
- It is slightly less dense than air.
- It is unstable and may explode when compressed into a liquid.
Chemical Properties of Ethyne
-
Combustion:
In air, ethyne combusts to form carbon(IV) oxide and water:
2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l)In limited air, it burns with a smoky, luminous flame due to high carbon content. In excess air, it burns with a hot, non-luminous flame (~3000°C).
-
Addition Reactions:
-
With Hydrogen
(Hydrogenation): In the
presence of nickel as a catalyst:
C2H2(g) + H2(g) → C2H4(g) → C2H6(g) -
With Halogens:
C2H2 + Cl2 → C2H2Cl2 → C2H4Cl2 -
With Hydrogen Halides:
C2H2 + HCl → CH=CHCl → CH2CHCl2 -
With Water (Hydration): In
the presence of dilute
H2SO4 and
HgSO4 catalyst, ethyne forms
ethanal:
C2H2 + H2O → CH3CHO -
With Acidified
KMnO4:
Decolourises acidified KMnO4, forming acetic acid and CO2 under vigorous oxidation.
-
With Hydrogen
(Hydrogenation): In the
presence of nickel as a catalyst:
-
Polymerization:
In the presence of a nickel-based catalyst, ethyne polymerizes to form benzene:
3C2H2 → C6H6 -
Substitution Reactions:
-
With ammoniacal copper(I)
chloride: Forms reddish-brown
copper(I) dicarbide:
C2H2 + 2CuCl → Cu2C2 + 2HCl -
With ammoniacal silver
nitrate: Forms white silver
dicarbide:
C2H2 + 2AgNO3 → Ag2C2 + 2HNO3
These reactions distinguish ethyne from ethene.
-
With ammoniacal copper(I)
chloride: Forms reddish-brown
copper(I) dicarbide:
Uses of Ethyne
- Mixed with oxygen to produce oxy-ethyne flame for cutting and welding metals.
- Used in the production of PVC plastics.
- Serves as a fuel in miner’s lamps.
- Used in the manufacture of synthetic fibres.
Test for Unsaturation
Unsaturated compounds such as ethyne decolourise bromine water.
Aromatic Hydrocarbons
Aromatic hydrocarbons are compounds that have structures similar to benzene. Benzene, the simplest aromatic compound, has the molecular formula C6H6.
Structure of Benzene
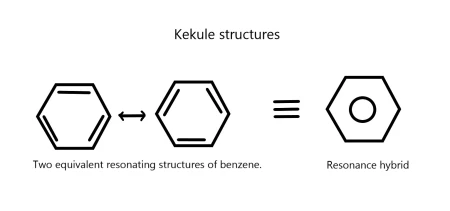
There was once much debate over benzene's structure. In 1865, August Kekulé proposed that benzene consists of a ring of six carbon atoms with alternating single and double bonds.
 Credit: madover
chemistry
Credit: madover
chemistry
These two alternating forms are called resonance structures. Resonance occurs when molecules have the same atomic arrangement but different electron arrangements. Benzene's resonance gives it extra stability.
Although Kekulé’s structure explained benzene's stability, it failed to explain why benzene does not undergo typical alkene addition reactions (like decolorizing bromine water or reacting with hydrogen halides).
Instead, benzene primarily undergoes substitution reactions. This observation led to a new model: the six π electrons in benzene are delocalized over all six carbon atoms, forming a cloud above and below the ring.
The modern structure of benzene is shown as a hexagon with a circle inside, representing this delocalized electron cloud.
Preparation of Benzene
- From Coal Tar: Benzene can be obtained from the destructive distillation of coal, which produces coal tar containing benzene.
- From
Petroleum: Through a process called
catalytic reforming, benzene can be
produced by dehydrogenating hexane using vanadium(V)
oxide (V2O5) at 500°C and 20
atm.
C6H14 → C6H6 + 4H2 - From Ethyne (Polymerization): Three
molecules of ethyne (acetylene) combine to form
benzene.
3HC≡CH → C6H6
Physical Properties of Benzene
- It has a pleasant, aromatic smell.
- It boils at 80°C.
- It is insoluble in water but soluble in organic solvents.
- It burns with a sooty flame due to high carbon content.
Chemical Properties of Benzene
Benzene shows unique reactivity due to its stable aromatic ring. In most of its chemical reactions, the benzene ring remains intact while substitutions or additions occur at the hydrogen atoms attached to the ring.
-
Stability of the Benzene Ring:
In most reactions involving benzene and its derivatives, the benzene ring (nucleus) remains unchanged.
-
Addition of Hydrogen:
Benzene behaves like an unsaturated compound and can undergo addition reactions. When hydrogen gas is passed over benzene in the presence of finely divided nickel at about 180°C, cyclohexane is formed.
C6H6 + 3H2 → C6H12 -
Addition of Halogens:
Benzene reacts with chlorine or bromine in the presence of sunlight or ultraviolet light to form an addition compound. With chlorine, benzene forms benzene hexachloride (BHC), also known as gammaxane, a white solid used as an insecticide.
C6H6 + 3Cl2 → C6H6Cl6 -
Reaction with Ozone:
When ozonised oxygen is bubbled into benzene at room temperature, it forms an unstable triozonide compound with the formula C6H6(O3)3.
-
Reaction with Ethene:
In the presence of heat, pressure, and anhydrous aluminium(III) chloride as catalyst, benzene reacts with ethene to form ethylbenzene.
C6H6 + CH2=CH2 → C6H5C2H5 -
Substitution Reactions:
Benzene typically undergoes substitution reactions where one or more hydrogen atoms on the ring are replaced by other atoms or groups.
-
Chlorination: Chlorine gas
reacts with benzene in the presence of a
chlorine carrier (e.g., iodine, red
phosphorus, or iron) to form chlorobenzene.
C6H6 + Cl2 → C6H5Cl + HCl - Bromination: Benzene reacts with bromine in the presence of iron(III) catalyst to form bromobenzene.
-
Nitration: A nitrating
mixture (concentrated HNO3 and
H2SO4) reacts with
benzene to form nitrobenzene. This reaction
is exothermic.
C6H6 + HNO3 → C6H5NO2 + H2OFurther substitution can lead to:
- Dinitrobenzene:
C6H4(NO2)2 - Trinitrobenzene:
C6H3(NO2)3
These nitro compounds are used in making explosives and dyes.
- Dinitrobenzene:
-
Chlorination: Chlorine gas
reacts with benzene in the presence of a
chlorine carrier (e.g., iodine, red
phosphorus, or iron) to form chlorobenzene.
-
Sulphonation:
When benzene is refluxed with concentrated H2SO4 for several hours, sulphonation occurs, forming benzene sulphonic acid. The reaction is faster with fuming sulphuric acid (oleum), and heating is not required.
C6H6 + H2SO4 → C6H5SO3H + H2O
Uses of Benzene
- It is used as a solvent for dissolving organic compounds.
- It is used as an additive in petrol (gasoline) to improve fuel efficiency.
- It is used in the manufacture of aromatic compounds, such as benzoic acid.