Fats and oils
Fats and oils naturally occur in living organisms and can be obtained from two main sources:
- Animal Tissues: Marine animals such as fish provide fish oil and cod-liver oil, while land animals supply products like butter and lard.
- Fruits and Seeds of Plants: These yield edible fats and oils, commonly known as vegetable oils. Examples include cocoa butter, palm oil, coconut oil, cottonseed oil, olive oil, corn oil, groundnut oil, sunflower oil, and soybean oil.
Structure and Types of Fats and Oils
Fats and oils are complex mixtures made up of esters formed from glycerol—a trihydric alkanol—and fatty acids. These esters are known as glyceryl trialkanoates. The alkyl group (R) from the fatty acids can either be the same or different in each molecule.
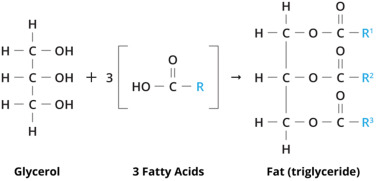 Credit: Sciencedirect
Credit: Sciencedirect
Fatty acids can be classified into two main types:
- Saturated fatty acids: These contain only single carbon-carbon bonds in their hydrocarbon chains.
- Unsaturated fatty acids: These contain one or more carbon-carbon double bonds in their hydrocarbon chains.
Fats contain a higher proportion of esters from saturated fatty acids, while oils contain more esters from unsaturated fatty acids.
Fats are considered simple lipids and are typically solid at room temperature.
Physical Properties
- Pure fats and oils are either white or lightly colored and almost odorless.
- They lack sharp melting or boiling points due to being mixtures of multiple triglycerides (esters).
- They are insoluble in water but dissolve in organic solvents such as alcohols.
- When heated above 300°C, they decompose and release irritating fumes.
Hydrogenation of Oils
Natural oils primarily consist of triglycerides formed from unsaturated fatty acids. These oils are liquids and not suitable for consumption in their natural form. To make them edible, they are hydrogenated to form solid fats.
Margarine is made from a refined blend of animal fats and vegetable oils. This mixture is purified using activated charcoal to remove color and odor and is neutralized to eliminate free acids. Hydrogen gas is bubbled through it at about 200°C and under 2–5 atmospheres of pressure using nickel as a catalyst, producing a more solid product.
Reaction:
Fats/Oils + H2(g) (Ni catalyst, 200°C, 2 atm) → Margarine
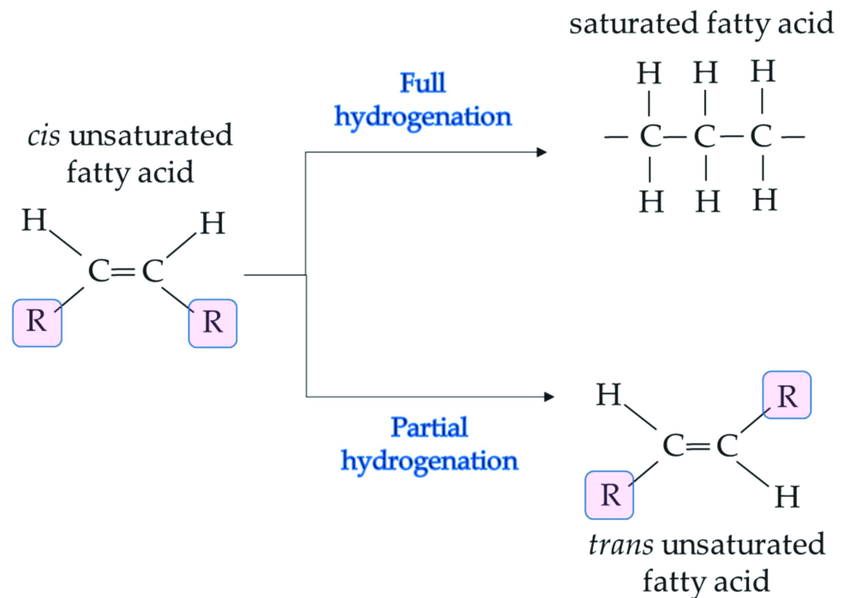 Credit: Researchgate
Credit: Researchgate
The hardened fats are then combined with salt, vitamins, skimmed milk, and other fats to produce margarine.
Saponification
Saponification is the process of boiling fats or oils with an alkali (e.g., NaOH or KOH) to produce soap (a salt of fatty acids) and a by-product known as propane-1,2,3-triol (glycerol), which is used in making paints.
Reaction:
Fat/Oil + 3NaOH → Glycerol (CH2OH–CHOH–CH2OH) + 3 RCOONa (soap)
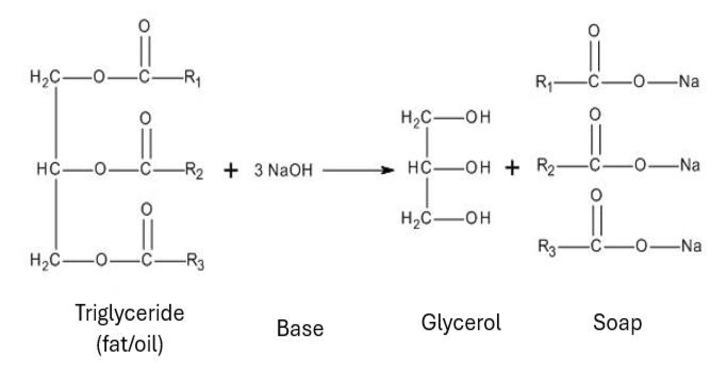 Credit: Researchgate
Credit: Researchgate
Acid Hydrolysis of Fats and Oils
When fats or oils are heated with water in the presence of concentrated H2SO4, they undergo hydrolysis to produce glycerol and a mixture of saturated and unsaturated fatty acids. These can be separated by fractional distillation.
Uses of Fats and Oils
- They are widely present in foods and serve as nutrients for growth and energy when metabolized, releasing carbon dioxide.
- They are used industrially to make soaps, glycerol, and margarine.
- They act as solvents in paints and are used in manufacturing products like candles and varnishes.
Soaps and detergents
Soap and Its Preparation
Soaps are salts of long-chain carboxylic acids, typically formed from the reaction of fatty acids with sodium or potassium hydroxide. These fatty acids are usually derived from natural fats and oils, which are triglycerides—esters of glycerol.
Traditional Preparation of Soap
Traditionally, soap is made by leaching wood ashes with water to create a strong alkaline solution. This basic solution is then heated with oil or fat. Soap is formed as a result of this reaction. Village soap makers rely on experience to determine when enough oil has been added to create good-quality soap. Often, the alkaline solution is evaporated to dryness before being used.
Commercial and Home Soap-Making Processes
The basic method of soap production is the same in both commercial and home settings, differing mainly in scale and the purity of the materials used. In the commercial process, fats and oils are boiled with aqueous sodium hydroxide until hydrolysis is complete. Sodium chloride (common salt) is then added to the mixture.
This addition of salt causes the soap to separate from the mixture in a process known as salting out. The soap floats to the top, while the by-product, glycerol, is removed by distillation. Perfumes, dyes, and disinfectants may be added to make toilet soaps. Fillers like sodium carbonate and sand can also be included before the soap is cut into bars.
Local Production of Black Soap
Locally, an alkaline solution is prepared by mixing water with ashes obtained from burnt plantain peels. This solution is then boiled with palm oil or palm kernel oil (PKO) to produce the traditional black soap commonly used in many communities.
Detergents
A detergent is any substance that helps to emulsify and remove grease, making it a cleansing agent. Soap is a type of detergent and is often referred to as a soapy detergent. However, the term detergent typically refers to synthetic substitutes for soap, also known as soapless detergents.
Soapless detergents are synthetic products made primarily from petroleum-based hydrocarbons, though they can also be derived from natural sources like fats and oils. These detergents are generally sodium or potassium salts of sulfonic acids. For example, alkylbenzenes are sulfonated using fuming sulfuric acid to produce alkylbenzene sulfonic acids, which are then neutralized with sodium hydroxide to form their sodium salts.
Note: Soapless detergents with branched hydrocarbon chains are not biodegradable.
Soapy Detergents
Soapy detergents refer to traditional soaps, which are made by the hydrolysis of fats and oils. They are biodegradable, especially when the molecules have unbranched alkyl chains.
Soap vs Hard Water
Hard water contains dissolved ions like Fe³⁺, Ca²⁺, and Mg²⁺, which react with soap to form insoluble scum. This wastes soap, discolors fabrics, and makes materials stiff. On the other hand, soapless detergents do not form scum because their sulfonates of Fe³⁺, Ca²⁺, and Mg²⁺ are soluble in water.
Cleansing Action of Soap
Dirt usually sticks to materials through grease, which consists of long-chain, non-polar hydrocarbons. Water is a polar solvent and cannot dissolve or remove greasy dirt on its own.
Soap molecules are amphipathic, meaning they have both polar and non-polar parts:
- Non-polar tail (R = C₁₇H₃₅): dissolves in grease or oil
- Polar head (-COO⁻Na⁺): dissolves in water
When soap dissolves in water, it dissociates into ions. The polar head interacts with water, while the non-polar tail embeds in the grease. This breaks the grease into smaller droplets and suspends them in water, effectively cleaning the material.
Advantages of Soap Over Detergents
- Soaps are biodegradable; many detergents are not.
- Unlike soaps, detergents may form protective envelopes around organic matter in wastewater, preventing natural decomposition.
Differences Between Soaps and Detergents
| Soap | Detergent |
|---|---|
| Cannot be used in acidic solutions (they break down in acidic conditions) | Can be used in acidic solutions (they are salts of strong acids) |
| Biodegradable and deteriorate with age | Non-biodegradable if they contain branched chains; do not deteriorate with age |
| Not very soluble in water | Highly soluble in water |
| Weaker cleansing action compared to detergents | Stronger cleansing action due to surfactants |
| Salts of long-chain fatty acids and alkali | Salts of sulfonic acids and alkali |
| Do not lather well with hard water | Lather easily with hard, soft, or salt water |