Alkanols (Alcohols)
Alkanols are a homologous series of organic compounds with the general molecular formula \( C_nH_{2n+1}OH \) or \( ROH \). They contain the hydroxyl (\( -OH \)) functional group.
Nomenclature
Alkanol names are derived by replacing the “e” in the name of the corresponding alkane with “ol”.
- Methanol – \( CH_3OH \)
- Ethanol – \( CH_3CH_2OH \)
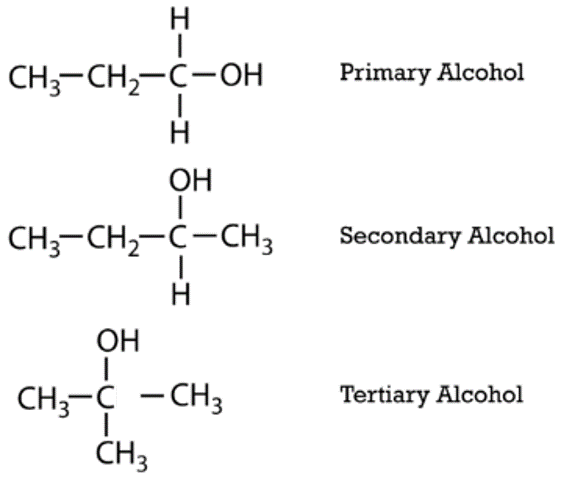
Classification of Alkanols
Alkanols are classified based on the number of alkyl groups attached to the carbon atom bearing the hydroxyl group.
- Primary Alkanols (1°): One alkyl group is attached to the carbon atom carrying the hydroxyl group.
- Secondary Alkanols (2°): Two alkyl groups are attached to the carbon atom bearing the hydroxyl group.
- Tertiary Alkanols (3°): Three alkyl groups are attached to the carbon atom bearing the hydroxyl group.
 Credit: Libretexts
Credit: Libretexts
Types of Alkanols
The type of alkanol depends on the number of hydroxyl groups in the molecule:
- Monohydric Alkanols: Contain one hydroxyl group. e.g., \( C_2H_5OH \), \( C_3H_7OH \)
- Dihydric Alkanols: Contain two hydroxyl groups.
- Polyhydric Alkanols: Contain three or more hydroxyl groups.
Ethanol
Laboratory Preparation
- Hydrolysis of ethyl esters using hot alkali.
- Reduction of ethanol with nascent hydrogen.
Commercial Preparation
1. From Ethene
Ethene from petroleum cracking reacts with concentrated sulfuric acid at 80°C and 30 atm:
\( C_2H_4 + H_2SO_4 \rightarrow C_2H_5HSO_4 \)
The product is hydrolyzed to form ethanol:
\( C_2H_5HSO_4 + H_2O \rightarrow C_2H_5OH + H_2SO_4 \)
Ethanol is distilled, and sulfuric acid can be reused.
2. By Fermentation
Ethanol can be produced industrially from starch- or sugar-rich raw materials via fermentation using yeast.
Production from Starchy Foods
- Crush and cook the starchy material (e.g., maize, rice).
- Extract starch with water and allow to settle.
- Treat starch with malt (contains the enzyme diastase) at 50°C.
- Starch is converted to maltose:
\( 2(C_6H_{10}O_5)_n + nH_2O \rightarrow nC_{12}H_{22}O_{11} \)
- Add yeast, which contains:
- Maltase: Converts maltose to glucose
\( C_{12}H_{22}O_{11} + H_2O \xrightarrow{maltase} 2C_6H_{12}O_6 \)
- Zymase: Converts glucose to ethanol and
carbon dioxide
\( C_6H_{12}O_6 \xrightarrow{zymase} 2C_2H_5OH + 2CO_2 \)
- Maltase: Converts maltose to glucose
Physical Properties of Ethanol
- Colourless and volatile liquid.
- Miscible with water.
- Boiling point of 78°C.
- Neutral to litmus paper.
Chemical Properties of Ethanol
1. Combustion
Ethanol burns in air with a clean flame:
\( 2CH_3OH + 3O_2 \rightarrow 2CO_2 + 4H_2O \)
2. Oxidation of Alkanols
The oxidation products of alkanols depend on their classification:
- Primary alkanols: First oxidized to alkanals (aldehydes), then further to alkanoic acids in the presence of an oxidizing agent like potassium manganate(VII) (\( KMnO_4 \)).
\( CH_3CH_2OH \xrightarrow{[O]} CH_3CHO \xrightarrow{[O]} CH_3COOH \)
- Secondary alkanols: Oxidized to alkanones (ketones).
- Tertiary alkanols: Do not undergo oxidation easily because there is no hydrogen atom on the carbon bearing the hydroxyl group.
Note: Colour changes of oxidizing agents:
- Purple \( KMnO_4 \) becomes colourless.
- Orange \( K_2Cr_2O_7 \) turns green.
3. Esterification
Esterification is a reversible reaction between an alkanol and an alkanoic acid to form a sweet-smelling ester. This reaction is catalyzed by concentrated sulfuric acid (\( H_2SO_4 \)).
\( CH_3CH_2OH + CH_3COOH \xrightarrow{H^+} CH_3COOCH_2CH_3 + H_2O \)
4. Dehydration of Alkanols
Alkanols can be dehydrated to form alkenes in the presence of concentrated \( H_2SO_4 \).
\( CH_3CH_2OH + H_2SO_4 \rightarrow CH_3CH_2HSO_4 + H_2O
\)
\( CH_3CH_2HSO_4 \xrightarrow{170^\circ C} C_2H_4 + H_2SO_4
\)
5. Reaction with Sodium and Potassium
Sodium and potassium react vigorously with alkanols to release hydrogen gas and form the corresponding organic salts.
\( 2C_2H_5OH + 2Na \rightarrow 2C_2H_5ONa + H_2 \)
6. Reaction with Phosphorus Halides
- With phosphorus pentachloride (\( PCl_5 \)):
- With phosphorus trichloride (\( PCl_3 \)):
\( C_2H_5OH + PCl_5 \rightarrow C_2H_5Cl + POCl_3 + HCl \)
\( 3C_2H_5OH + PCl_3 \rightarrow 3C_2H_5Cl + H_3PO_3 \)
Uses of Ethanol
- Used as an organic solvent.
- Main component of methylated spirit, used for cleaning wounds and dissolving paint.
- Added to petrol as a fuel additive for vehicles.
- Used in the manufacture of chemicals such as ethanoic acid and esters.
- Used as an ingredient in alcoholic beverages like beer, wine, and spirits.
- Used as antifreeze in car radiators due to its low freezing point (\( -117^\circ C \)).