Solutions and Solubility
A solution is a uniform or homogeneous mixture of two or more substances.
Solution = Solute + Solvent
Definitions
- Solute: The substance that is dissolved. It may be a solid, liquid, or gas.
- Solvent: The substance that dissolves the solute, usually a liquid.
Types of Solutions
- Aqueous Solution: A solution where the solvent is water.
- Chemical Solution: A solution formed with a chemical change. For example, when magnesium is added to dilute hydrochloric acid, it forms magnesium chloride, which then dissolves in water.
True and False Solutions
- True Solution: A solution where solute particles fully dissolve and fit between solvent particles. Example: Aqueous sodium chloride or copper(II) tetraoxosulphate(VI).
- False/Colloidal Solution: The solute particles are larger than in true solutions but still invisible to the naked eye. Examples include starch and albumen.
Types of Colloids
- Sols and Gels: Solids dispersed in liquids (e.g., starch, glue, jelly).
- Aerosols: Liquids dispersed in gases (e.g., fog, smoke, insecticide sprays).
- Emulsions: Liquids dispersed in other liquids (e.g., milk, hair cream). Detergents work due to their ability to form emulsions.
Solubility
Solubility is the maximum amount of solute (in moles or grams) that dissolves in 1 dm³ of solvent to form a saturated solution at a given temperature.
It is expressed in mol/dm³ or g/dm³.
Solubility Equations
Solubility in mol/dm³ = \( \frac{\text{Concentration in g/dm}^3}{\text{Molar mass}} \)
Solubility in g/dm³ = \( \frac{\text{Mass} \times 1000}{\text{Volume}} \)
Solubility in mol/dm³ = \( \frac{\text{Mass} \times 1000}{\text{Molar mass} \times \text{Volume}} \)
Effect of Temperature
- The solubility of solids generally increases with rising temperature.
- The solubility of gases generally decreases with rising temperature.
Definition of Terms
- Saturated Solution: A solution that contains the maximum amount of solute at a given temperature, with some undissolved solute present.
- Unsaturated Solution: A solution that contains less solute than it can dissolve at a given temperature.
- Supersaturated Solution: A solution that contains more solute than it can normally dissolve at a given temperature.
Factors Affecting Solubility
- Nature of the solute and solvent
- Temperature
- Pressure (mainly affects gases)
Solubility Curves
These are graphs that show the relationship between solubility and temperature.
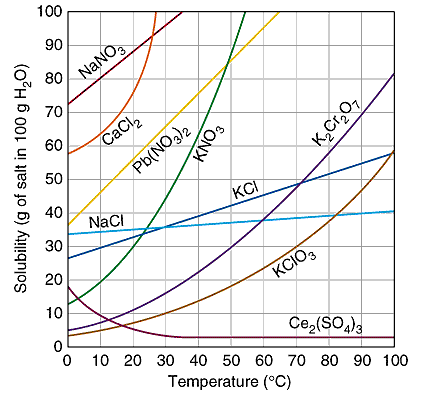 Credit: sciencegeek
Credit: sciencegeek
Uses of Solubility Curves
- To identify suitable solvents and temperatures for extracting substances from natural sources.
- To determine the best temperature for separating a mixture of salts through fractional crystallization.
- To help pharmacists calculate the quantity of a solid drug needed in a solvent to prepare a given mixture.
Determining the Solubility of a Solid in Water at a Given Temperature
- Place distilled water in a beaker and heat it to a temperature slightly above room temperature (around 296 K).
- Add the solid salt gradually with constant stirring until no more dissolves — this indicates a saturated solution has been formed.
- Allow the saturated solution to cool to room temperature.
- Use a pipette to measure a known volume of the saturated solution and transfer it into a clean, dry, pre-weighed evaporating dish.
- Weigh the evaporating dish containing the solution.
- Evaporate the water using a water bath or sand bath until the solution is dry.
- Allow the dish to cool and then reweigh it.
- Repeat heating and weighing until a constant mass is obtained.
- Calculate the number of moles of salt in the known volume, and then calculate the solubility in 1 dm³ of the saturated solution.
Solubility Calculation Formula
Solubility in mol/dm³ = \( \frac{\text{Mass of salt} \times 1000}{\text{Molar mass} \times \text{Volume of solution in cm}^3} \)
Solubility Rules in Water
- All compounds of Group 1 metals (Li⁺, Na⁺, K⁺, etc.) are soluble in water.
- All ammonium (NH₄⁺) compounds are soluble.
- All nitrates (NO₃⁻) are soluble.
- All chlorides (Cl⁻), bromides (Br⁻), and iodides (I⁻) are soluble, except those of Ag⁺, Hg₂²⁺, and Pb²⁺.
- All sulphates (SO₄²⁻) are soluble, except those of Sr²⁺, Ba²⁺, and Pb²⁺. CaSO₄ and Ag₂SO₄ are sparingly soluble.
- All carbonates (CO₃²⁻), phosphates (PO₄³⁻), and chromates (CrO₄²⁻) are insoluble, except those of Group 1 metals and ammonium.
- All hydroxides (OH⁻) and oxides (O²⁻) are insoluble, except those of Group 1 metals and ammonium. Ca²⁺, Sr²⁺, and Ba²⁺ hydroxides are sparingly soluble.
- All sulphites (SO₃²⁻) and sulphides (S²⁻) are insoluble, except those of Group 1 metals and ammonium.
Note: These rules help predict whether a precipitate will form during qualitative analysis.
Solubility Product Constant (Ksp)
Some salts, like barium sulphate (BaSO₄), are only sparingly soluble in water. When placed in water, a small number of Ba²⁺ and SO₄²⁻ ions dissociate into solution. An equilibrium is established between the solid salt and its dissolved ions.
Equilibrium Expression
The equilibrium expression for the dissolution of BaSO₄ is:
\( \text{BaSO}_4(s) \rightleftharpoons \text{Ba}^{2+}(aq) + \text{SO}_4^{2-}(aq) \)
Since this is a heterogeneous equilibrium (involving solids and aqueous ions), the solid is not included in the equilibrium expression. The solubility product is:
\( K_{sp} = [\text{Ba}^{2+}][\text{SO}_4^{2-}] \)
Important Notes
- 1. The solubility product applies only to sparingly soluble ionic compounds.
- 2. It cannot be used for compounds that are readily soluble (e.g., NaCl) due to ion interactions in solution.
- 3. The units of Ksp depend on the specific equilibrium expression and must be calculated case by case.
- 4. Ksp only applies to saturated solutions.