Fluids at rest
Density of a Substance
The density of a substance is defined as its mass per unit volume:
$$ \text{Density} = \frac{\text{Mass of the substance}}{\text{Volume of the substance}} $$
Density is a scalar quantity and is measured in kilograms per cubic meter (kg·m-3).
Determination of Density
To determine the density of a substance, its mass and volume must be measured. The mass can be found using a weighing scale, and the volume can be determined using a graduated density bottle.
Relative Density (R.D)
Relative density (R.D) of a substance is the ratio of the mass or weight of any volume of the substance to the mass or weight of an equal volume of water. It can be expressed in different forms:
Mathematical Expressions of Relative Density
- Using mass: $$ R.D = \frac{\text{Mass of the substance}}{\text{Mass of an equal volume of water}} $$
- Using weight: $$ R.D = \frac{\text{Weight of the substance}}{\text{Weight of an equal volume of water}} $$
- Using density: $$ R.D = \frac{\text{Density of the substance}}{\text{Density of water}} $$
- Using weight in air and water: $$ R.D = \frac{\text{Weight of object in air}}{\text{Weight of an equal volume of water}} $$
- Using upthrust in water: $$ R.D = \frac{\text{Weight of object in air}}{\text{Upthrust of the object in water}} $$
- Using upthrust in any liquid: $$ R.D = \frac{\text{Upthrust of the object in liquid}}{\text{Upthrust of the object in water}} $$
Relative Density of Liquids
Relative density of a liquid can be determined using the upthrust and displaced volume:
- $$ R.D = \frac{\text{Upthrust of object in water}}{\text{Volume of liquid displaced by the object}} $$
- $$ R.D = \frac{\text{Volume of water displaced by the same object}}{\text{Volume of liquid displaced by the object}} $$
Archimedes' Principle
The equilibrium of bodies in a fluid is governed by Archimedes' principle and the principle of floatation. These concepts are closely related to the densities and relative densities of objects and fluids.
Archimedes' Principle
Archimedes' principle states that when an object is completely or partially immersed in a fluid, it experiences an upthrust equal to the weight of the fluid displaced by the object.
Principle of Floatation
The principle of floatation states that an object will float when the upthrust exerted by the fluid is equal to the weight of the object.
Upthrust of an Object in a Fluid
The upthrust experienced by an object submerged in a fluid is given by:
\[ U_p = W_A - W_F \]Where:
- \( U_p \) = Upthrust
- \( W_A \) = Weight of the object in air
- \( W_F \) = Weight of the object in the fluid
If the fluid is water, gas, or any other liquid, then the upthrust can be expressed as:
\[ U_p = W_w - W_L \]Expression for Upthrust
The upthrust experienced by an object in a fluid is also equal to the weight of the displaced fluid:
\[ U_f = \text{Mass of displaced fluid} \times g \] \[ U_f = \text{Density of fluid} \times \text{Volume of displaced fluid} \times g \] \[ U_f = \rho \times V \times g \]Where:
- \( U_f \) = Upthrust
- \( \rho \) = Density of the fluid
- \( V \) = Volume of the displaced fluid
- \( g \) = Acceleration due to gravity
Flotation
When a body is immersed or floating in a liquid, two forces act on it:
- Weight (\( W \)): Acts downward due to gravity.
- Upthrust (\( U \)): Acts upward due to the displaced fluid.
If the upthrust is equal to the object's weight, the object floats. However, if the object's weight is greater than the upthrust, it sinks.
Principle of Floatation
The principle of floatation states that when a body is fully or partially immersed in a fluid, its weight is equal to the weight of the fluid displaced:
\[ W_{\text{body}} = W_{\text{fluid displaced}} \]Why Objects Like Ships and Ice Float
- They displace a volume of water whose weight is equal to their own weight.
- Their weight is in equilibrium with the upthrust exerted by the fluid.
Fluids at Rest and in Motion
The molecules in gases move more freely than those in solids. Since both liquids and gases can flow, they are classified as fluids.
Surface Tension
Surface tension is the force of attraction between liquid molecules that makes the liquid behave like an elastic skin. It can also be defined as the force per unit length acting on the liquid surface at a right angle to an imaginary line drawn on the surface.
Effects of Surface Tension
- Water droplets dripping from a tap form a spherical shape.
- Mercury forms spherical pellets when poured on a surface.
- A greased needle floats when gently placed on water.
Factors That Reduce Surface Tension
- Adding soap or detergents
- Heating
- Contamination
Mathematical Expression for Surface Tension
Surface tension (r) is given by:
$$ r = \frac{F}{2L} $$
where:
- r = Surface tension
- F = Force
- L = Length
SI Unit: Newton per meter (N·m-1)
Application of Surface Tension
- Waterproof materials (e.g., umbrellas, raincoats)
- Cleansing action of soaps and detergents
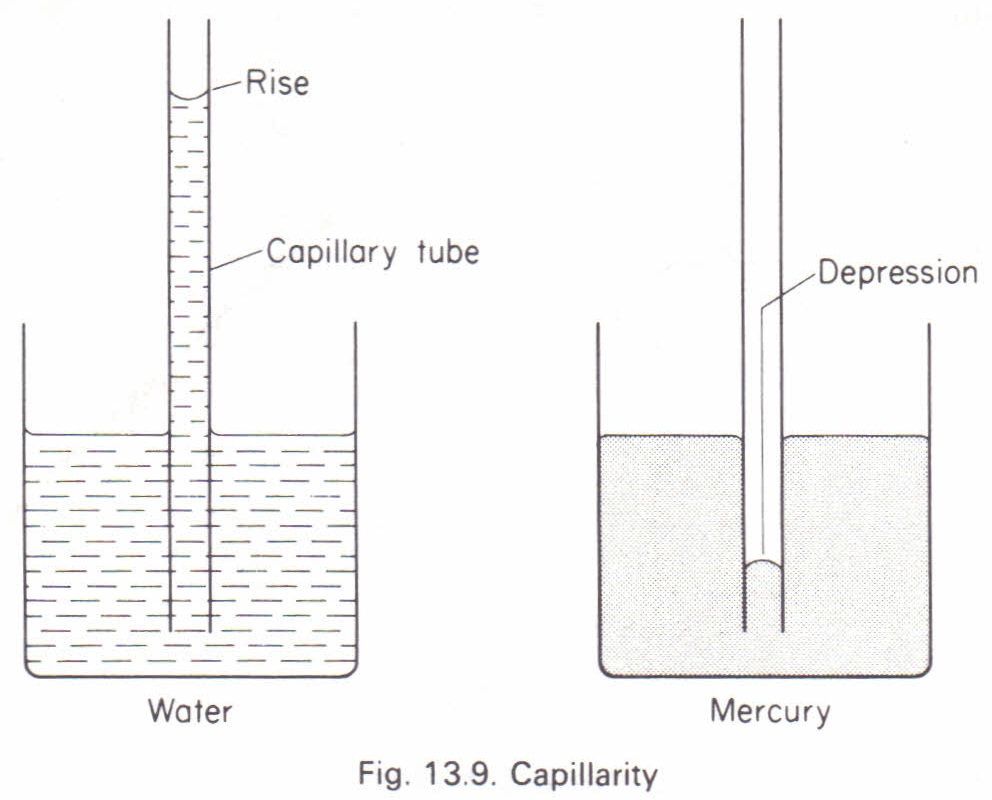
Capillarity
Capillarity (or capillary action) is the tendency of a liquid to rise or fall in a narrow tube when the tube is dipped into the liquid. The rise of the liquid depends on:
- The cross-sectional area of the tube
- Surface tension
 Credit: PhysicsMax
Credit: PhysicsMax
When one end of a thin glass capillary tube is immersed in a liquid like water, the liquid rises higher in the tube than in the container. This occurs because the adhesive forces between the glass and water are stronger than the cohesive forces between water molecules.
In contrast, when mercury is placed in a glass tube, the cohesive forces between mercury molecules are greater than the adhesive forces between mercury and glass. As a result, the mercury level is lower inside the tube.
The phenomenon of capillarity is utilized in absorbent materials such as blotting paper, towels, cotton wool, and wicks. It also plays a role in the upward movement of water from the soil through plant roots and stems.
Cohesion
Cohesion is the force of attraction between molecules of the same kind. For example, the cohesion between mercury molecules prevents mercury from wetting a glass surface.
Adhesion
Adhesion is the force of attraction between molecules of different substances. For example, the adhesion between water molecules and glass causes water to wet a glass surface.